The main topic of the research activities was the chemistry and physics of highly photostable fluorescent dyes. Novel fluorescent dyes were prepared by new synthetic procedures and their properties were studied. Such dyes are for example of interest for dye laser applications or for light-collecting systems. Furthermore, various analysis methods could be developed on the basis of these dyes, for example, for the immunochemical determination of traces. This was applied for the analysis of pollutants in aquatic systems. Optical fluorescence memory systems were established with such dyes and have been further developed.
As well as these dye specific investigations there were various reaction mechanistic studies, for example the quantification of solvent effects in chemical reactions in binary mixtures and reaction mechanisms of rearrangement reactions. Other topics concern polymer chemical subjects.
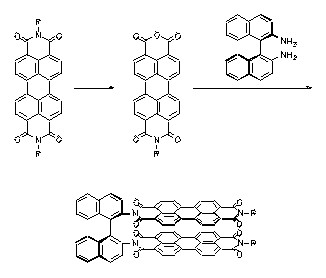
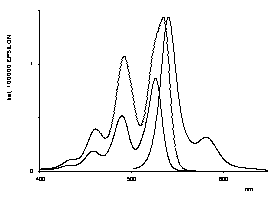
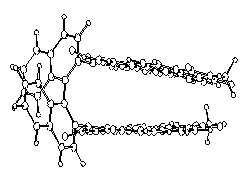
Bichromophoric perylene dyes were prepared where the two chromophores are linked by a chiral binaphthyl unit. A strong exciton coupling induced a bathochromic shift of UV/Vis absorption and fluorescence and extraordinarily strong CD effects. These newly prepared dyes are model compounds for the photosynthesis reaction center.
 |
 |
 |
 |
 |
Natural and artificial |
UV/Vis- and CD-spectra of the artificial |
· Heinz Langhals, Josef Gold, Liebigs Ann./Recueil, 1997, 1151-1153. J. Karolin, L. B.-Å. Johansson, U. Ring, H. Langhals, Spectrochim. Acta A 1996, 52, 747-753.
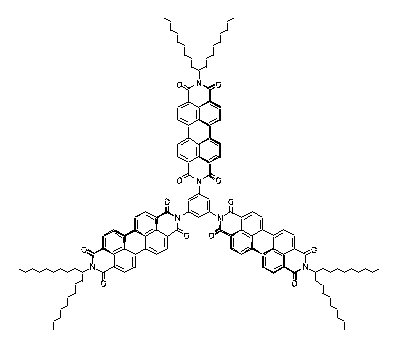
Three perylene chromophores were linked in m-position of a benzene ring so that through-bond interactions were minimal and through-space interactions could be studied. A slight bathochromic shift of the absorption of the interacting chromophores was observed and indicates a destabilizing effect of such type of interactions. This is of interest for the "longicyclics" problem; the latter has been frequently investigated with the highly strained hydrocarbon barrelene.
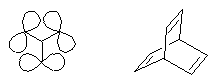
Longicyclics · · · · · · · Barrelene |
 |
· Heinz Langhals, Josef Gold, J. Prakt. Chem. 1996, 338, 654-659.
Perylene-3,4-dicarboxylic anhydride is an important intermediate for the synthesis of dyes and was prepared with an entropy controlled one-step synthesis with 25% yield from technical perylene-3,4:9,10-bisanhydride by partial decarboxylation employing non-condensing amines. Perylene-3-carboxylic acid and perylene-3,4-dicarboxylic imide were prepared in the same way in yields of 24% and 76%, respectively.
 |
· Heinz Langhals, Petra von Unold, Markus Speckbacher, Liebigs Ann./Recueil 1997, 467-468.
Very soluble, lightfast and intensely red fluorescent dyes were prepared by the condensation of perylene-3,4:9,10-tetracarboxylic bisanhydride and perylene anhydride imides, respectively, with geminal long-chain alkyl 1,3-diamines.
 |
· Heinz Langhals, Haleh Bastani-Oskoui, J. Prakt. Chem. 1997, 339, 597-602.
Pyrrolo- and thiophenoperylenedicarboximides were prepared by a reductive cyclisation of corresponding nitro compounds and are strongly fluorescent heterocycles. These can be easily monofunctionalized and used for fluorescence labelling.
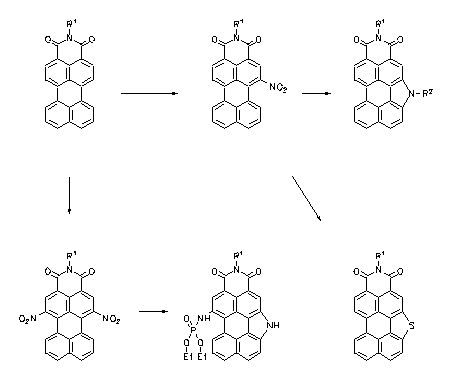 |
· Heinz Langhals, Leonhard Feiler, Liebigs Ann. 1996, 1587-1591.
A novel type of rearrangement of aromatic bisimides was found. This reaction is useful for the preparation of aromatic amino acids. The novel rearrangement was verified with perylene and naphthalene bisimides and novel highly fluorescent dyes could be obtained. An experimental basis for the loose-bolt mechanism for fluorescence quenching could be established using these novel compounds.
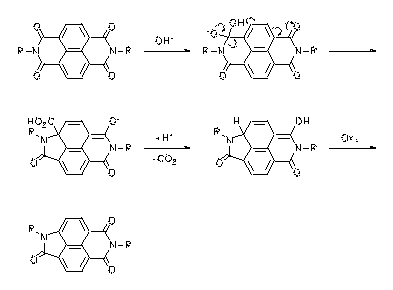 |
· Heinz Langhals, Petra von Unold, GIT Fachz. Lab. 1997, 41, 974-978. H. Langhals, P. v. Unold, Angew. Chem. 1995, 107, 2436-2439; Angew. Chem., Int. Ed. Engl. 1995, 34, 2234-2236.
Oxindigo has never attained technical importance in contrast to its N analog (indigo) and S analog (thioindigo) because of its hypsochromic absorption (413 nm in cyclohexane), low molar absorptivity (13800), lack of fluorescence and difficult preparation. Oxindigo derivatives are subject of only few publications. However, a substitution in the conjugating positions 6 and 6' with donor groups results in bathochromically absorbing dyes with a pronounced fluorescence in solution (see figue). In contrast to indigo the cis-derivative absorbs even more bathochromically than the trans derivative, forms blue solutions and exhibits a solid-state fluorescence in the NIR.
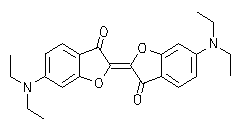 |
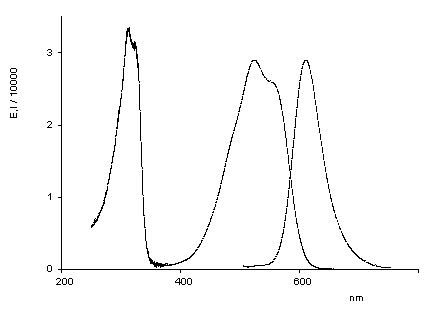 UV/Vis absorption and fluorescence spectra of trans-oxindigo in CHCl3 (color cordinates: x = 0.3791, y = 0.2169, z = 0.4040 at Tmax= 0.1). |
In contrast to the literature oxindigo derivatives could not be prepared from cumaranone and disulphur dichloride because of the formation of a tetrathiane. However, oxindigo derivatives can be prepared from halogenated acetic acid derivatives and corresponding phenols.
 |
 |
· Heinz Langhals, Barbara Wagner, Angew. Chem. 1996, 108, 1090-1093; Angew. Chem. Int. Ed. Engl. 1996, 35, 1016-1019. H. Langhals, B. Wagner, K. Polborn, Tetrahedron, 1996, 52, 1961-1964.
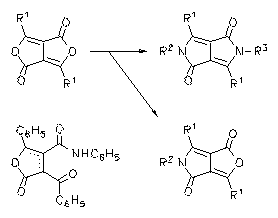
N-arylated DPP dyes were prepared by the condensation of the corresponding lactames with arylamines with the support of additives such as dicyclohexyl cabodiimide. They form bright-red solids with a red to orange solid-state fluorescence.
 |
 |
· H. Langhals, T. Grundei, T. Potrawa, K. Polborn, Liebigs Ann. 1996, 679-682.
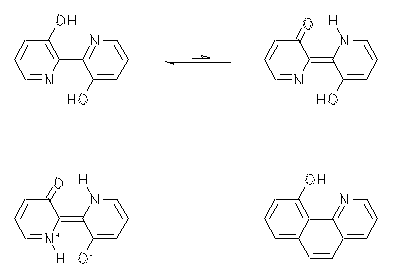
2,2'-Bipyridine-3,3'-diol is a fluorescent dye of special interest because of its large Stokes-shift of 100 nm. It has been claimed that an intramolecular two-proton-transfer after the optical excitation according to the ESIPT mechanism is responsible. The resulting tautomer was said to be stable only in the excited state because of the lack of an energy minimum in the electronic ground-state. The best experimental proof of this statement was a very weak solvatochromism of the dye both in absorption and emission. This fact was taken as an indicator for point symmetry in the ground and excited state of the dye.
However, in contrast to the literature an additional weak UV/Vis absorption was found that is typical for tautomers and this absorption becomes even stronger in more polar media. Moreover, a comparably weak solvatochromism was found for a structurally related phenantridine derivative, however, only a one-proton-transfer is possible for the latter. Therefore one must conclude that the experimental support for a two-proton-transfer for 2,2'-bipyridine-3,3'-diol is exceptionally weak.
 |
· L. B.-Å. Johansson, L. Persson, H. Langhals, J. Chem. Soc., Faraday Trans., 1996, 92, 4909-4911.
1. H. Langhals, P. von Unold, 'Arylimid-Lactam-Ringverengungsreaktionen - ein neuer Typ von Umlagerungen', GIT Fachz. Lab. 1997, 41, 974-978.
2. H. Langhals, J. Gold, 'Chiral Bifluorophoric Perylene Dyes with Unusually High CD Effects - a Simple Model for the Photosynthesis Reaction Center', Liebigs Ann./Recueil, 1997,1151-1153.
3. H. Langhals, P. von Unold, M. Speckbacher, 'Balanced Decarboxylation of Aromatic Polyacids - a One-Step Synthesis of Perylene 3,4-dicarboxylic Anhydride', Liebigs Ann./Recueil 1997, 467-468.
4. H. Langhals, H. Bastani-Oskoui, 'Synthesis of Readily Soluble Tetraazaviolanthrone and -isoviolanthrone Fluorescent Dyes', J. Prakt. Chem. 1997, 339, 597-602.
5. L. B.-Å:. Johansson, L. Persson, H. Langhals, 'Conspicuous absorption and fluorescence spectroscopic properties of 3,3'-dihydroxy-2,2'-bipyridines in solution', J. Chem. Soc., Faraday Trans., 1996, 92, 4909-4911.
6. J. Karolin, L. B.-Å. Johansson, U. Ring, H. Langhals, 'Aggregation of perylene dyes in lipid vesicles: the effect of optically active substituents', Spectrochim. Acta A 1996, 52, 747-753.
7. H. Langhals, L. Feiler, 'Pyrrolo- and Thiophenoperylenedicarboximides - Highly Fluorescent Heterocycles', Liebigs Ann. 1996, 1587-1591.
8. H. Langhals, J. Gold, 'Tangentially Coupled p Systems and their Through-Space Interaction - Trichromophoric Perylene Dyes', J. Prakt. Chem. 1996, 338, 654-659.
9. H. Langhals, B. Wagner, 'Oxindigo: Colour Deepening, Strong Fluorescence and Large Stokes-Shift by Donor-Substitution', Angew. Chem. 1996, 108, 1090-1093; Angew. Chem. Int. Ed. Engl. 1996, 35, 1016-1019.
10. H. Langhals, B. Wagner, K. Polborn, 'The Oxydative Coupling of Coumaranone with Disulfurbichloride', Tetrahedron 1996, 52, 1961-1964.
11. H. Langhals, T. Grundei, T. Potrawa, K. Polborn, 'Highly Photostable Organic Fluorescent Pigments - A Simple Synthesis of N-Arylpyrrolopyrrolediones (DPP)', Liebigs Ann. 1996, 679-682.
1. H. Langhals, W. Jona, 'Perylenfarbstoff-Kronenether: Fluoreszenz-Komplexbildner für Metallionen', Ger. Offen. DE 19709008.7 (March 5, 1997).
2. H. Langhals, W. Jona, 'Ein selbsdispergierender organischer Farbstoff', Ger. Offen. DE 19709004.4 (March 5, 1997).
3. H. Langhals, J. Gold, 'Bifluorophore Perylenfarbstoffe mit langwellig verschobener Fluoreszenz', Ger. Offen. DE 19702826.8 (January 27, 1997).
4. H. Langhals, P. von Unold, 'Die ausbalancierte Decarboxylierung von aromatischen Polycarbonsäuren - eine einstufige Synthese von Perylen-3,4-dicarbonsäureanhydrid', Ger. Offen. DE 19700990.5 (January 14, 1997).
5. H. Langhals, L. Feiler, 'Pyrrolo- und Thiophenoperylenimide - stark fluoreszierende Heterocyclen', Ger. Offen. DE 19651712.5 (December 12, 1996).
6. H.Langhals, B. Hock, R. A. Brosius, 'Die immunochemische Bestimmung von Azofarbstoffen', Ger. Offen. DE 19651599.8 (December 11, 1996).
7. H. Langhals, B. Wagner, 'Donor-substituted oxindigo derivatives and their use as colorants', Ger. Offen. DE 19616532.6 (April 25, 1996); PCT Int. Appl. WO 9741176; Chem. Abstr. 1998, 128, 4661a.
H. Langhals, 2009. - Impressum - Datenschutz - Kontakt