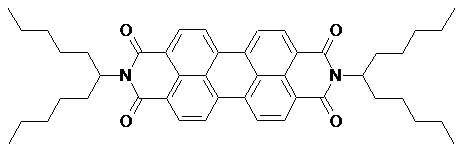
N,N‘-Bis(1-pentylhexyl)-3,4:9,10-perylenebis(dicarboximide);
2,9-bis-(1-pentylhexyl)anthra[2,1,9-def;6,5,10-d‘e‘f‘]diisoquinoline-1,3,8,10(2H,9H)-tetraone; 2,9-bis-(6-undecyl)anthra[2,1,9-def;6,5,10-d‘e‘f‘]diisoquinoline-1,3,8,10(2H,9H)-tetraone, RN 110590-83-5:
M.p. 183-184°C, red crystals. UV (CHCl3): λmax
(ε) = 459 nm (18580), 489.5 (52240), 526.5 (87500). C46H54N2O4 (689.9) calcd. C 79.05, H 7.79, N 4.01; found C 79.18, H 7.81, N 3.84.
References, RN 110590-83-5:
*1. Demmig, S.; Langhals, H. ‘Very soluble and photostable perylene fluorescent dyes’, Chem. Ber. 1988, 121, 225-230.
2. Langhals, H.; Demmig, S.; Huber, H. ‘Rotational barriers in perylene fluorescent dyes’, Spectrochim. Acta, Part A: Molecular and Biomolecular Spectroscopy 1988, 44A, 1189-93.
3. Langhals, H. ‘Lightfast, readily soluble perylenetetracarboxylic (bis)imide fluorescent dyes’, Ger. Offen. 1988, DE 3703495 A1 19880818.
4. Loehmannsroeben, H. G.; Langhals, H. ‘Laser performance of perylenebis(dicarboximide) dyes with long secondary alkyl chains’, Appl. Physics B: Photophysics and Laser Chemistry 1989, B48, 449-452.
5. Langhals, H. ‘(Polymeric) perylenetetracarboxylic diimide dyes having high dielectric constants’, Ger. Offen. 1989, DE 3814647 A1 19891005.
6. Langhals, H.; Potrawa, T. ‘Optical memory devices containing color changeable dyes, and dyes therefor’, PCT Int. Appl. 1990, WO 9001480 A1 19900222.
7. Langhals, H.; Demmig, S. ‘Perylene dyes and their use as permanent toner in electrophotography and laser printing’, Ger. Offen. 1991, DE 4007618 A1 19910912.
8. Schott, H.; Von Cunow, D.; Langhals, H. ‘Labeling of liposomes with intercalating perylene fluorescent dyes’, Biochim. Biophys. Acta, Biomembranes 1992, 1110, 151-157.
9. Singh, Th. B.; Erten, S.; Guenes, S.; Zafer, C.; Turkmen, G.; Kuban, B.; Teoman, Y.; Sariciftci, N. S.; Icli, S. ‘Soluble derivatives of perylene and naphthalene diimide for n-channel organic field-effect transistors’, Organic Electronics 2006, 7, 480-489.
10. Ebel, A.; Donaubauer, W.; Hampel, F.; Hirsch, A. ‘Amphiphilic pyrene-functionalized dendrons: Synthesis and intermolecular interactions’, Europ. J. Org. Chem. 2007, 3488-3494.
11. Tuerkmen, G.; Erten-Ela, S.; Icli, S. ‘Highly soluble perylene dyes: Synthesis, photophysical and electrochemical characterizations’, Dyes and Pigments 2009, 83, 297-303.
12. Langhals, H.; Pust, T. ‘Perylenetetracarboxylic diimide dye-containing micellar nanoparticles and applications’, Ger. Offen. 2010, DE 102009008661 A1 20100819.
13. Erten-Ela, S.; Turkmen, G. ‘Perylene imide dyes for solid-state dye-sensitized solar cells: Spectroscopy, energy levels and photovoltaic performance’, Renewable Energy 2011, 36, 1821-1825.
14. Langhals, H.; Pust, T, ‘Lipophilic optical supramolecular nano devices in the aqueous phase’, Green and Sustainable Chem. 2011, 1, 1-6.
15. Schmidt, C. D.; Lang, N.; Jux, N.; Hirsch, A. ‘A facile route to water-soluble coronenes and benzo[ghi]perylenes’, Chem. Europ. J. 2011, 17, 5289-5299, S5289/1-S5289/9.
16. Boobalan, G.; Imran, P. M.; Nagarajan, S. ‘Self-assembly, optical and electrical properties of fork-tailed perylene bisimides’, Superlattices and Microstructures 2012, 51, 921-932.
17. Do, Jung Yun; Jang, Byungkwon ‘The efficient synthesis of N-fused coronene analogs and a related polyimide with near-infrared absorption’, Polymer Journal (Tokyo, Japan) 2013, 45, 1177-1182.
18. Zhong, Yu; Kumar, Bharat; Oh, Seokjoon; Trinh, M. Tuan; Wu, Ying; Elbert, Katherine; Li, Panpan; Zhu, Xiaoyang; Xiao, Shengxiong; Ng, Fay; et al. ‘Helical Ribbons for Molecular Electronics’, J. Am. Chem. Soc. 2014, 136, 8122-8130.
19. Jiang, Wei; Ye, Long; Li, Xiangguang; Xiao, Chengyi; Tan, Fang; Zhao, Wenchao; Hou, Jianhui; Wang, Zhaohui ‘Bay-linked perylene bisimides as promising non-fullerene acceptors for organic solar cells’, Chem. Comm. (Cambridge, United Kingdom) 2014, 50, 1024-1026.
20. Madhurima, V.; Priyanka, K. Greeshma; Pavan, K. S. N. D.; Nagarajan, S. ‘Analysis of wetting of perylene diimide thin films-on-glass by water’, Key Engineering Materials 2014, 594-595(Advanced Materials Engineering and Technology II, 1074-1077.
21. Boobalan, G.; Imran, P. K. M.; Manoharan, C.; Nagarajan, S. ‘Optical and electrical properties of new perylene diimide thin films’, J. Electronic Materials 2015, 44, 4000-4005.
22. Ye, Long; Sun, Kai; Jiang, Wei; Zhang, Shaoqing; Zhao, Wenchao; Yao, Huifeng; Wang, Zhaohui; Hou, Jianhui ‘Enhanced efficiency in fullerene-free polymer solar cell by incorporating fine-designed donor and acceptor materials’, ACS Applied Materials & Interfaces 2015, 7, 9274-9280.
23. Meng, Dong; Fu, Huiting; Xiao, Chengyi; Meng, Xiangyi; Winands, Thorsten; Ma, Wei; Wei, Wei; Fan, Bingbing; Huo, Lijun; Doltsinis, Nikos L.; et al. ‘Three-bladed rylene propellers with three-dimensional network assembly for organic electronics’, J. Am. Chem. Soc. 2016, 138, 10184-10190.
24. Weintraub, M. T.; Xhakaj, E.; Austin, A.; Szarko, J. M. ‘The effects of donor : acceptor intermolecular mixing and acceptor crystallization on the composition ratio of blended, spin coated organic thin films’, J. Materials Chem. C: Materials for Optical and Electronic Devices 2016, 4, 7756-7765.
25. Zhao, Xiaohong; Xiong, Yushuai; Ma, Jie; Yuan, Zhongyi ‘Rylene and rylene diimides: comparison of theoretical and experimental results and prediction for high-rylene derivatives’, J. Phys. Chem. A 2016, 120, 7554-7560.
26. Yang, Chuluo; Zhang, Chen ‘Preparation of benzothiophene or benzoselenophene fused perylene imide derivatives useful as receptor in organic solar cells’, Faming Zhuanli Shenqing 2016, CN 105906652 A 20160831.
27. Lin, Zhi; Li, Cheng; Meng, Dong; Li, Yan; Wang, Zhaohui ‘Hybrid corannulene-perylene dyes: Facile synthesis and optoelectronic properties’, Chem. An Asian J. 2016, 11, 2695-2699.
28. Meng, Dong; Sun, Dan; Zhong, Chengmei; Liu, Tao; Fan, Bingbing; Huo, Lijun; Li, Yan; Jiang, Wei; Choi, Hyosung; Kim, Taehyo; et al. ‘High-performance solution-processed non-fullerene organic solar cells based on selenophene-containing perylene bisimide acceptor’, J. Am. Chem. Soc. 2016, 138, 375-380.
29. Jackson, N. E.; Chen, L. X.; Ratner, M. A. ‘Charge transport network dynamics in molecular aggregates’, Proc. of the National Academy of Sciences of the United States of America 2016, 113, 8595-8600.
30. Guo, Zongxia; Yu, Ping; Sun, Kai; Wang, Wenpin; Wei, Yuhan; Li, Zhibo ‘Two-dimensional crystallization of rylene diimide-based n-type semiconductors tuned by the dimensions of the aromatic core at the liquid-solid interface’, Chem. An Asian J. 2017, 12, 1104-1110.
31. Gao, Guangpeng; Liang, Ningning; Geng, Hua; Jiang, Wei; Fu, Huiting; Feng, Jiajing; Hou, Jianhui; Feng, Xinliang; Wang, Zhaohui ‘Spiro-fused perylene diimide arrays’, J. Am. Chem. Soc. 2017, 139, 15914-15920.
32. Park, G. E.; Choi, S.; Lee, D. H.; Godumala, M.; Uddin, M. A.; Woo, H. Y.; Cho, M. J.; Choi, D. H. ‘Perylene diimide isomers containing a simple sp3-core for non-fullerene-based polymer solar cells’, J. Materials Chem. A: Materials for Energy and Sustainability 2017, 5, 663-671.
33. Guo, Y.; Li, Y.; Awartani, O.; Han, H.; Zhao, J.; Ade, H.; Yan, H.; Zhao, D. ‘Improved performance of all-polymer solar cells enabled by naphthodiperylenetetraimide-based polymer acceptor’, Adv. Materials (Weinheim, Germany) 2017, 29, DOI:10.1002/adma.201700309.
34. Zhong, Y.; Sisto, T. J.; Zhang, B.; Miyata, K.; Zhu, X.-Y.; Steigerwald, M. L.; Ng, F.; Nuckolls, C. ‘Helical nanoribbons for ultra-narrowband photodetectors’, J. Am. Chem. Soc. 2017, DOI:10.1021/jacs.6b13089.
35. Hu, Yu; Chen, Shixiao; Zhang, Lifu; Zhang, Youdi; Yuan, Zhongyi; Zhao, Xiaohong; Chen, Yiwang ‘Facile approach to perylenemonoimide with short side chains for nonfullerene solar cells’, J. Org. Chem. 2017, 82, 5926-5931.
36. Fan, Y.; Ziabrev, K.; Zhang, S.; Lin, B.; Barlow, S.; Marder, S. R. ‘Comparison of the optical and electrochemical properties of bi(perylene diimide)s linked through ortho and bay positions’, ACS Omega 2017, 2, 377-385.
37. Li, X.; Wang, H.; Schneider, J. A.; Wei, Z.; Lai, W.-Y.; Huang, W.; Wudl, F.; Zheng, Y. ‘Catalyst-free one-step synthesis of ortho-tetraaryl perylene diimides for efficient OPV non-fullerene acceptors’, J. Materials Chem. C: Materials for Optical and Electronic Devices 2017, 5, 2781-2785.